3. Continuous Manufacturing
SMBR
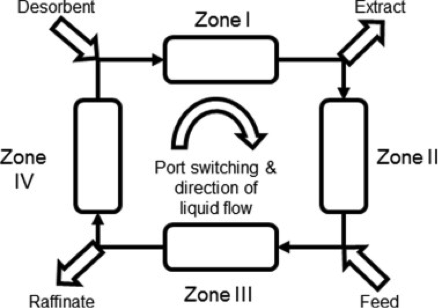
Schematic of a standard configuration SMBR operation.
Simulated moving bed reactor (SMBR) is a continuous and simultaneous reaction and separation operation. It is a derivative of the simulated moving bed (SMB) chromatography, which was developed as a continuous method of liquid chromatography over 50 years ago. SMB has been widely used in industry for the separation of sugars, petrochemicals, and pharmaceuticals. Combined with reaction, the SMBR is a powerful tool for use in reactions that are conversion limited and separations that need higher resolution. The challenges in SMBR is determining the optimal operations. A paper by Tie. et al (link) describes a model-based approach to simultaneous determine the model parameters to accurately describe the SMBR process and the operating conditions for its operation.
Additional work is ongoing to examine how the SMBR operation fits into an overall plant process that includes downstream processing operations. Different configurations, standard and superstructure is considered when analyzing how the recycle stream from the downstream process can be reintegrated into the SMBR.
Crystallization
The pharmaceutical industry has been moving towards continuous crystallization in recent years thanks to pressure from the FDA to fully understand and control the processes used to make drugs. Soon the recipe-based processes of the past (e.g. first cool reaction products to temperature T, next add X weight percent of antisolvent A, and then filter-off finished product) will no longer be acceptable. Continuous crystallization promises higher throughput and consistent product quality. Reactive crystallization has the ability to crystallize products via the supersaturation generated by the reaction, forming high-purity products that can easily be separated. In the Bommarius Group we use enzymes to drive the reaction and crystallize beta-lactam antibiotics. In paring continuous crystallization with reactive crystallization we hope to increase the availability of high-quality beta-lactam antibiotics. One of the biggest challenges associated with continuous enzymatic reactive crystallization is simultaneously optimizing two processes in one vessel; for example, increasing the concentration of the reactants speeds the reaction but slows crystallization. Despite these challenges, the Bommarius Group has shown that pairing these two phenomena in a continuous fashion is actually beneficial to the process as a whole; showing better selectivity and yield than individual and batch-wise reaction and crystallization.
